Is Mixing Nacl and Agno3 a Chemical Change
Happens when you mix nacl and agno3 answers com balance chemical equation online balancer chemistry lab copper silver nitrate reaction silver. For example suppose that one adds a solution of Na 2 SO 4 to another solution which is known to contain either NaCl MgCl 2.

Double Displacement Reaction Of Agno3 And Nacl Youtube
Allow it to return to room temperature in a desiccator this will we done for you.
. Although this gas is evidence of a chemical reaction neither of the indicated products is a gas. That means AgNO 3 and NaCl is a precipitation reaction. When aqueous AgNO 3 and aqueous NaCl compounds are mixed together there is a high chance of giving a white colour precipitate if initial silver nitrate and initial sodium chloride concentrations are considerably high.
The evolution of heat is evidence of a chemical reaction. Precipitate a color change the evolution of heat and the evolution of a gas. Mass of NaCl 398 Kg Mass of water 100 Kg Moles of Nacl mass of NaCl MW of NaCl 398 Kg.
1 chemical reactions do not always go to completion and 2 a state of dynamic equilibrium can be established in a chemical system by the simultaneous formation of products from reactants and reactants from. Solution formed but individual properties retained. Identify all of the phases in your answer.
Separation possible by distillation. This precipitate is definite evidence of a chemical reaction. Silver nitrate is an inorganic compound with the chemical formula AgNO3.
It is used in making photographic films and in laboratory setting as a staining agent in protein visualization in PAGE. Pour down the sink and wash the well plate. A simple demonstration of how a precipitate is evidence of a chemical reaction taking place is performed by mixing solutions of calcium chloride and sodium carbonate to form the precipitate calcium carbonate CaCO 3.
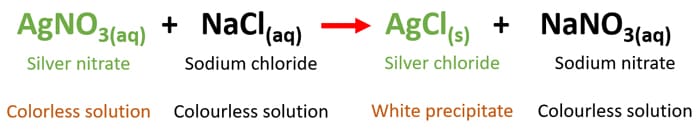
White precipitate or new substance is formed. NaCl AgNO 3 à NaNO 3 AgCl The formulas of the products are obtained by switching the ions A white precipitate is produced when these solutions are mixed. This is intended as an introductory activity involving the concept of dynamic equilibrium.
Such a change indicates that a new chemical compound has been formed and the change in properties can be used to identify unknown solutions. When you mix nacl and agno3 answers com chemistry lab demonstrations silver nitrate copper wire observation when cu is mixed with agno3 chemical reaction with color change slnova org mix between naoh cuso4 and food making color orange when i react zn with cuso4 i get cu znso4 but what is the gas that is formed during this reaction a flame test. Added to the aqueous solution of sodium chloride NaCl a white precipitate of silver chloride AgCl is formed that is indicated by the following chemical reaction.
If we touched the container it feels warm. D mixing NaCl and AgNO3. Retaining of properties no new substances created f adding HCL to Mg chemical reaction.
B burning paper chemical change gas and heat given off evidence of chemical reaction c dissolving NaCl physical change. ScienceChemistryQA LibraryCorrect the incorrect reaction AgNO3aqNaClaqNaClsAgNO3aqAgNO3aqNaClaqNaClsAgNO3aq Express your answer as a chemical equation. 3 2 HCl 2 NaCl H 2CO 3 Bubbles of a colorless gas are evolved when these solutions are mixed.
Neither is a gas or producer of gas. NaOHaq HClaq NaClaq H 2 Oliq Does a reaction happen. In its solid form silver nitrate is coordinated in a trigonal planar arrangement.
Mixing NaCl and AgNO3 - chemical change. The hydrogen ions H from the acid and the hydroxide ion OH from the. Observation When Cu Is Mixed With Agno3 chemical reaction with color change slnova org chemistry unit 7 lab copper silver nitrate reaction how can the reaction kbr agno3 gt agbr kno3 be essay on.
AgNO3 NaCl ---. NaCl and H 2 O are soluble so no precipitate. AgNO3aq NaClaq Æ AgCls NaNO3aq Sodium chloride NaCl remains in solution meaning that exits as a sodium ion Na and a chloride ion Cl-.
In this section you will be asked to find two pairs of ionic solutions the WILL form a precipitate when mixed and two pairs of ionic solutions that WILL NOT form a precipitate when mixed. How much of a 290 M hydrochloric acid solution would you dilute to prepare 5000 mL of 850 M HCl. If 002 M AgNO 3 is to be used weigh a 015-020.
Exothermic reaction by release of heat which is evidence of a chemical reaction. If you mix NaCl and AgNO3 the new possible combinations are NaNO3 and AgCl. The ideas developed in this activity are.
A tearing paper physical change. One of the two products sodium nitrate NaNO 3 or silver chloride AgCl is insoluble. H 2CO 3 H 2O CO 2 g Therefore CO 2 and H.
PROCEDURE Dry the NaCl sample at 110C for about 1 hr. But carbonic acid H 2CO 3 is an unstable compound and readily decomposes into carbon dioxide and water. It results in the formation of the precipitate of AgCl.
CaCl 2 aq Na 2 CO 3 aq CaCO 3 s 2NaClaq. Cut a small piece of Mg Magnesium ribbon. Correct the incorrect reaction.
It is often used as a precursor to other silver-containing compounds. Dichlorofluorescein in a solution prepared by mixing 75 mL of ethanol and 25 mL of water. Add a micro spatula of NaCl Sodium Chloride to a well in the well plate.
NaNO3 is soluble but AgCl is insoluble so you would predict that AgCl would precipitate. Mix it with about 3-4 drops of water and stir it with a tooth pick. Retaining of properties no new substances created.
Mixing salt NaCl with AgNO3 is a chemical change. In this tutorial we will learn about different aspects of this reaction such as precipitating and required. E tearing Mg ribbon physical change.
Add 2 drops of AgNO3 Silver nitrate to the mixture that you just made in the previous step.
Agno3 Nacl Agcl Nano3 Silver Nitrate Sodium Chloride Reaction
What Is The Product Formed When Agno3 Reacts With Nacl Quora

Precipitation Reaction Agno3 Nacl Youtube

Reaction Of Nacl Sodium Chloride And Agno3 Silver Nitrate Then Ammonia Youtube
Comments
Post a Comment